On March 31, the ImmunizeBC website will move over to HealthLinkBC.ca After this date, you will be automatically redirected to HealthLink BC’s Immunization landing page. HealthLink BC provides trusted health information online and over the phone 24 hours a day, seven days a week by calling 8-1-1.
Date last reviewed:
Thursday, Dec 05, 2024
On this page:
Information
Key points
- The ingredients in vaccines are important for keeping them safe and effective. They are safe in the amounts used.
- Some people worry that certain vaccine ingredients, like aluminum, gelatin, and formaldehyde might be harmful. But this is only true if they are present in much larger amounts than what is found in vaccines. Even water can be dangerous in large enough amounts.
- It’s important to know that each ingredient is used only in very small amounts, and only necessary ingredients are included. The ingredients are carefully tested during safety studies.
Types of vaccine ingredients
Learn about the different types of ingredients in vaccines, why they are used, and examples by clicking on the tabs below.
- Antigens
-
Antigens are the active ingredients in vaccines because they are the part that trigger an immune response (the body’s way of fighting germs). Antigens are usually killed or weakened viruses or bacteria, or parts of them. Some COVID-19 vaccines provide instructions for the body to produce the antigen rather than containing the antigen itself.Did you know? Although the number of vaccines children receive has increased in recent years, the number of antigens in vaccines has significantly decreased.
- Preservatives
-
Some vaccines contain very small amounts of preservatives to prevent harmful germs from growing in them. Examples of these preservatives include phenol, 2-phenoxyethanol, and thimerosal.
- Thimerosal is a preservative that contains a form of mercury called ethylmercury, which is different from the type of mercury (methylmercury) found in the environment. Ethylmercury is broken down and removed from the body much faster than methylmercury and is, therefore, much less likely to build up and cause harm. Most routine childhood vaccines in Canada don't contain thimerosal, except for some influenza vaccines. Thimerosal has been used safely in vaccines for many years, and research shows no evidence that it causes harm.
- Adjuvants
-
Adjuvants, like aluminum salts and squalene, are added to some vaccines to boost the body’s immune response. Without adjuvants, people might need more or higher doses of vaccines to achieve protection.
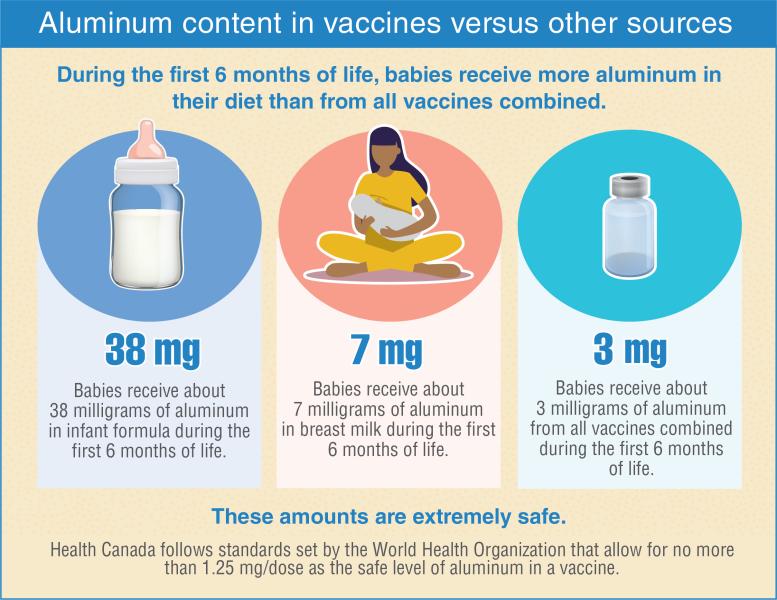
- Aluminum is found in air, food, and water, and it’s also present in breast milk and infant formula in amounts similar to those in vaccines. Once in the bloodstream, aluminum is processed the same, whether injected through vaccines or taken in through food. Hundreds of millions of people have been safely vaccinated with aluminum-containing vaccines.
- Squalene is a naturally occurring substance found in plants, animals, and humans, as well as in certain foods and cosmetics. It is made by the liver and circulates throughout the bloodstream.
- Additives
-
Additives, such as gelatin, human serum albumin, or bovine-derived reagents, are included in vaccines to keep them effective during storage.
- The gelatin used in vaccines is the same substance found in many foods, such as Jello, marshmallows, and gummy bears. Severe allergic reactions to gelatin are extremely rare, occurring in about 1 case per 2 million doses.
- Human blood products are not typically found in vaccines, with two exceptions: the rabies vaccines Imovax® Rabies and RabAvert®, which contain albumin derived from human blood. While there is an extremely small theoretical risk of infectious agents being present in human blood products, steps in the manufacturing process of both human albumin and human albumin-containing vaccines eliminate the risk of transmission of these agents. There have been no documented cases of vaccine-related transmission of infectious agents by human serum albumin.
- In Canada, some vaccines are made using bovine-derived reagents (materials sourced from cows). These ingredients come from cows known to be free of bovine spongiform encephalopathy, also known as "mad cow disease." The risk that currently licensed vaccines contain prions—the proteins responsible for variant Creutzfeldt-Jakob disease, the human form of mad cow disease—is zero.
- Substances from the manufacturing process
-
Some substances from the manufacturing process, such as formaldehyde, antibiotics, egg proteins, or yeast proteins, may remain in very small amounts in the final product.
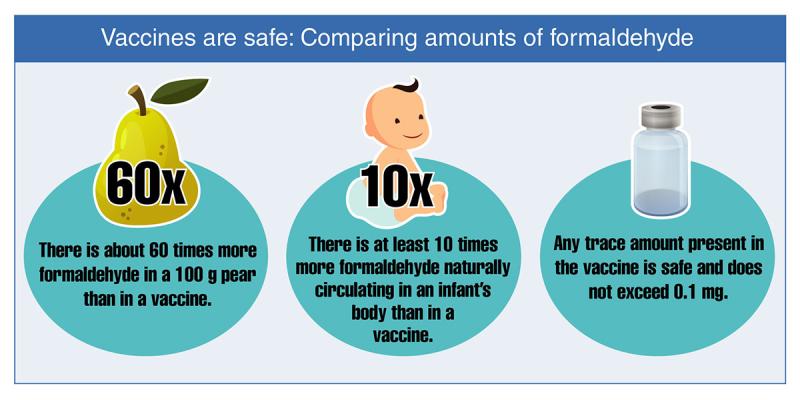
- Formaldehyde is often used to kill or weaken the virus or bacteria used to make a vaccine and is removed during the manufacturing process. Any trace amounts that may be left in the vaccine are safe. Formaldehyde is naturally produced in the body and helps with metabolism. There is about ten times more formaldehyde in an infant's body at any given time than is found in a vaccine.
- Antibiotics are used in some vaccines to prevent bacterial contamination during production. Most of these antibiotics are removed during the purification process, but trace amounts may remain in some vaccines. The types of antibiotics that are most likely to cause immediate allergic reactions (such as penicillin) are not used in vaccines.
- Egg proteins may be present in some vaccines because chicken eggs or cells are used to grow the viruses used in those vaccines. Most of the egg protein is removed in the manufacturing process, but very small amounts may remain in the final product. People with egg allergies can safely receive routine vaccines.
- Yeast protein may be present in some vaccines because yeast is used in their production. While the vaccines are purified to remove nearly all the yeast, trace amounts may still be present in the final product.
- Some vaccines use human cell lines in the early stages of production, but the purification process removes nearly all the cell components so that only trace amounts of DNA and protein may be present in the vaccine. Fetal cells are used because viruses need to be grown in cells, and human cells are often better than animal cells at supporting the growth of human viruses. The fetal cells used for vaccines were obtained from abortions that happened decades ago, and those abortions were not done for vaccine production. These cells continue to grow in the lab, and no new fetal cells are used to make vaccines today.
Ingredients by vaccine
To find a list of ingredients for each vaccine, you can refer to the following resources:
- Part 1 of the Canadian Immunization Guide contains a table listing the ingredients of vaccines used in Canada.
- The Government of Canada website website provides detailed information on the ingredients in each COVID-19 vaccine.
- You can also access the ingredient lists in the product monograph for each vaccine, available through Health Canada's Drug Product Database.
- References
-
-
Offit PA, Jew RK. Addressing Parents’ Concerns: Do Vaccines Contain Harmful Preservatives, Adjuvants, Additives, or Residuals? Pediatrics [Internet]. 2003 Dec [cited 2021 Mar 4];112(6):1394-1401
-
Children’s Hospital of Philadelphia. Vaccine ingredients: What you should know [Internet]. Philadelphia (PA): Children’s Hospital of Philadelphia; 2020 [cited 2021 Mar 4] 2 p. Available from: https://media.chop.edu/data/files/pdfs/vaccine-education-center-vaccine-ingredients.pdf
-
Children’s Hospital of Philadelphia. Aluminum in vaccines: What you should know [Internet]. Philadelphia (PA): Children’s Hospital of Philadelphia; 2014 [cited 2021 Mar 4] Aluminum in vaccines: What you should know. Vaccine safety: Immune system and health. 2021 Jan 26 [cited 2021 Mar 4]. 2 p. Available from: https://media.chop.edu/data/files/pdfs/vaccine-education-center-aluminum.pdf
-
WHO Expert Committee on Biological Standardization. Annex 6: Recommendations to assure the quality, safety and efficacy of DT-based combination vaccines [Internet]. Geneva: World Health Organization. 2014 [cited 2021 Mar 4]. Available from: https://www.who.int/publications/m/item/annex-6-trs980-combined-vax
-
National Advisory Committee on Immunization. Canadian Immunization Guide [Internet]. Evergreen ed. Ottawa (ON). Public Health Agency of Canada. 2012 [updated 2020 Jan 30]. Part 1 – Key immunization information: Contents of immunizing agents available for use in Canada; [cited 2021 Mar 4]. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-1-key-immunization-information/page-15-contents-immunizing-agents-available-use-canada.html
-
Offit PA, Bell LM. Vaccines: What you should know. 3rd ed. Hoboken, NJ: John Wiley & Sons, Inc.; c2003. 239 p.
-
National Advisory Committee on Immunization. Thimerosal: Updated statement. Can Commun Dis Rep [Internet]. 2007 Jul [cited 2021 Mar 4];33:1-13. Available from: https://www.canada.ca/en/public-health/services/reports-publications/canada-communicable-disease-report-ccdr/monthly-issue/2007-33/thimerosal-updated-statement.html
-
Centers for Disease Control and Prevention. Understanding thimerosal, mercury and vaccine safety [Internet]. Atlanta (GA): U.S. Department of Health & Human Services; 2013 Feb [cited 2021 Mar 4]. 2 p. Available from: https://www.cdc.gov/vaccines/hcp/patient-ed/conversations/downloads/vacsafe-thimerosal-color-office.pdf
-
National Advisory Committee on Immunization. Canadian Immunization Guide [Internet]. Evergreen ed. Ottawa (ON). Public Health Agency of Canada. 2012 [updated 2021 Jan 8]. Part 2 – Vaccine safety: Contraindications, precautions and concerns; [cited 2021 Mar 4]. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-2-vaccine-safety/page-3-contraindications-precautions-concerns.html#a2
-
Canadian Albacore Tuna [Internet]. Victoria (BC): Canadian Highly Migratory Species Foundation; c2015. The facts about mercury 2015 Jul 24 [cited 2021 Mar 4]. Available from: http://canadianalbacoretuna.com/the-facts-about-mercury/
-
HealthLinkBC [Internet]. Victoria (BC): Government of British Columbia. Mercury in Fish; 2018 Dec [cited 2021 Mar 4]. Available from: https://www.healthlinkbc.ca/healthlinkbc-files/mercury-fish
-
Gold R. Your child’s best shot: A parent’s guide to vaccination. 3rd ed. Ottawa (ON): Canadian Paediatric Society; c2006. 370 p. Eldred BE, Dean AJ, McGuire TM, Nash AL. Vaccine components and constituents: responding to consumer concerns. Med J Aust [Internet]. 2006 Feb 20 [cited 2021 Mar 4];184(4):170-5.
-
Immunization Action Coalition [Internet]. Saint Paul (MN): Immunization Action Coalition. Moral reflections on vaccines prepared from cells derived from aborted human foetuses. 2005 Jun 9 [cited 2021 Mar 4] Available from: https://www.immunize.org/talking-about-vaccines/vaticandocument.htm
-
World Health Organization Expert Committee on Biological Standardization. World Health Organization Technical Report Series No. 932 [Internet]. Geneva: World Health Organization; 2005 [cited 2021 Mar 4]. 137 p. Available from: https://www.who.int/groups/expert-committee-on-biological-standardization
-
Children’s Hospital of Philadelphia. Vaccines and Mad-Cow Disease [Internet]. Philadelphia (PA): Children’s Hospital of Philadelphia; 2018 [cited 2024 November 25]. Available from: https://www.chop.edu/vaccine-education-center/vaccine-safety/vaccines-and-other-conditions/mad-cow-disease
-
National Advisory Committee on Immunization. Canadian Immunization Guide [Internet]. Evergreen ed. Ottawa (ON). Public Health Agency of Canada. 2012 [updated 2016 August]. Part 1 – Key immunization information: Communicating effectively about immunization; [cited 2024 November 25]. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-1-key-immunization-information/page-5-communicating-effectively-immunization.html
-